Progress#
Originally designed to simulate the life cycle of a single E. coli cell on a single GPU, Lattice Microbes was subsequently expanded to support multi-GPU execution, thereby accommodating larger organisms such as yeast and accelerating ensemble simulations [HALL2014]. These computational advances have now culminated in a landmark achievement: the first full whole-cell dynamical models of the minimal bacterium JCVI-syn3A. In these studies, fundamental cellular behaviors emerge directly from multi-scale simulations of a living minimal cell [THORNBURG2022]. By integrating cryo-electron tomography, genome-scale metabolic networks, and stochastic–deterministic hybrid methods, the model recapitulates transcription–translation coupling, multiple DNA replication events, genome-wide mRNA half-lives, and experimentally observed doubling behavior.
More recently, this framework has been extended to achieve the first in silico simulation of an entire 100-minute cell cycle in four dimensions, capturing genetic information processes, metabolism, growth, and division, with each replicate cell exhibiting unique stochastic trajectories [THORNBURG2025a]. Furthermore, the explicit incorporation of 21 essential macromolecular complexes—including RNA polymerase, ribosome, degradosome, ABC transporters, and ATP synthase—provides mechanistic insight into how assembly kinetics, metabolic constraints, and spatial organization shape cellular physiology [FU2025].
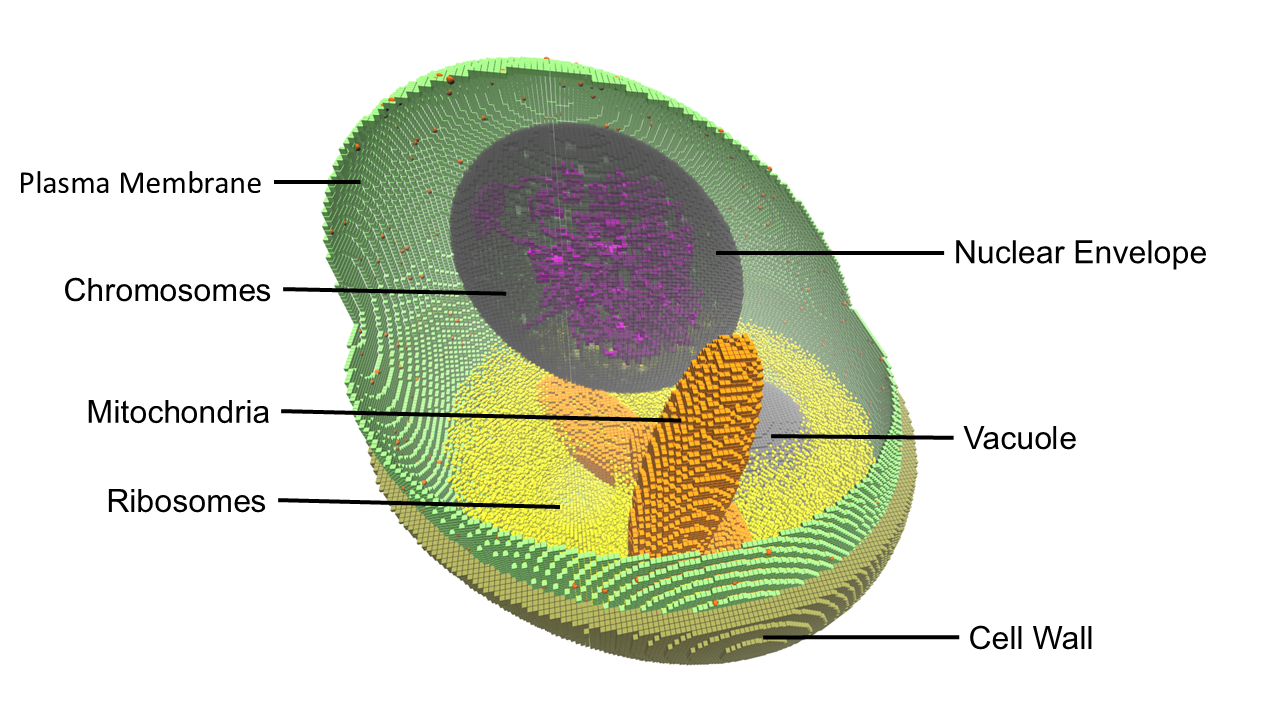
Beyond bacterial and minimal cell models, recent work has extended these approaches to eukaryotic systems. In a simplified model of the galactose switch in haploid Saccharomyces cerevisiae, a hybrid RDME–ODE framework was used to investigate how spatial heterogeneity and subcellular architectures influence gene regulation [WU2025]. Incorporating data-driven representations of chromosome positioning, endoplasmic reticulum organization, and ribosome localization revealed that these structural features markedly altered transporter production and intracellular galactose dynamics. Chromosome positioning increased variability across replicates without affecting mean expression, ER geometry constrained transporter delivery to the membrane, and restricting ribosomes to specific subdomains strongly suppressed Gal2p production and uptake. These findings demonstrate that explicitly accounting for spatial and architectural heterogeneity is essential for predicting eukaryotic cell behaviors, underscoring the broader applicability of Lattice Microbes–based simulations from minimal bacteria to the complex regulation of yeast. [WU2025].